United States
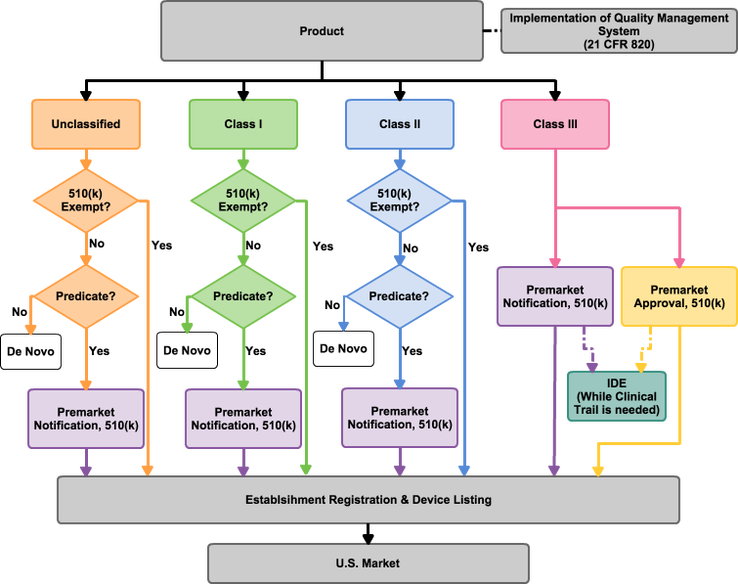
Medical devices planning to enter the U.S. market are required to classify in accordance with U.S. FDA regulations into Unclassified, Class I, Class II, and Class III. Each type of device is assigned a product code that indicate the rightful regulations to be followed. To sell medical devices in the U.S., devices are obligated to obtain clearance or approval from the FDA. Most Class II devices have to obtain clearance for Premarket Notification and 510(k). Class III devices require either clearance or approval through 510(k) or Premarket Approval (PMA). Normally, clinical trials are required for PMA, while only a few 510(k) submissions require clinical testing data in their submission. Sometimes devices with new intended uses or with specific and significant modifications might need supporting data from clinical trials. To perform clinical trails or tests, the procedure needs to be evaluated and approved by the Investigational Device Exemption (IDE) program. Once sufficient supportive information is submitted and reviewed, the FDA will decide whether the device is qualified for clearance or approval.
A 510(K) is a premarket submission made to the FDA to demonstrate that the device to be marketed is at least as safe and effective (substantially equivalent) to a legally marketed device that is not subject to PMA. Therefore, finding a predecessor is a key element of a 510(k). The most important part of the submission is a comprehensive comparison between the devices to its predecessor. In contrast, a PMA submission depends more on scientific references and tests to demonstrate that the device to be marketed is safe and effective in accordance to its claims. Choosing a submission requires entirely different strategies for applicants. AcmeBiotechs assists clients by preparing submissions in an economic and efficient way.
With clearance or approval, manufacturers can register their companies and list devices. Devices that are exempted from 510(k) and PMA can be sold in the U.S. market after completing establishment registration and device listing. As stated in U.S. medical device regulations, a U.S. Agent is obligatory for foreign companies during registration and device listing. U.S. Agents work as a communication window between the U.S. FDA and firms.
AcmeBiotechs has years of experience on regulatory consulting and QMS counseling for medical devices companies planning to enter theU.S. market. We help our clients prepare and submit device listings, establishment registrations, 510(k), PMA and IDE. Contact us for any questions related to accessing the U.S. medical device market.
A 510(K) is a premarket submission made to the FDA to demonstrate that the device to be marketed is at least as safe and effective (substantially equivalent) to a legally marketed device that is not subject to PMA. Therefore, finding a predecessor is a key element of a 510(k). The most important part of the submission is a comprehensive comparison between the devices to its predecessor. In contrast, a PMA submission depends more on scientific references and tests to demonstrate that the device to be marketed is safe and effective in accordance to its claims. Choosing a submission requires entirely different strategies for applicants. AcmeBiotechs assists clients by preparing submissions in an economic and efficient way.
With clearance or approval, manufacturers can register their companies and list devices. Devices that are exempted from 510(k) and PMA can be sold in the U.S. market after completing establishment registration and device listing. As stated in U.S. medical device regulations, a U.S. Agent is obligatory for foreign companies during registration and device listing. U.S. Agents work as a communication window between the U.S. FDA and firms.
AcmeBiotechs has years of experience on regulatory consulting and QMS counseling for medical devices companies planning to enter theU.S. market. We help our clients prepare and submit device listings, establishment registrations, 510(k), PMA and IDE. Contact us for any questions related to accessing the U.S. medical device market.