Taiwan
To commercialize medical devices in Taiwan, foreign companies must comply with the regulations by the Taiwan Food and Drug Administration (TFDA). The TFDA classifies medical devices into Class I, II, or III in accordance with their risk levels. The TFDA classification system is similar to U.S. FDA classification system. Class I devices have lower risk, Class II and Class III devices have higher risk. Premarket approval is required for all classes of medical devices prior to entering the Taiwanese market.
To apply for premarket approval, foreign medical device manufacturers are required to appoint an in-country representative, also known as a Taiwan Agent, to manage their device registration process and communicate with the Taiwan Food and Drug Administration (TFDA) on their behalf. A Taiwan Agent must have a local address in Taiwan and hold a formal license for medical device wholesale, medical device retail or international business. A Taiwan Agent should be responsible for submitting all documentation required for device approval and registration processes.
During the approval application, medical companies need to have quality system documents, known as QSD, to prove that the manufacturing steps comply with the FDA Quality System Regulation or ISO 13485:2003. This is a quality system standard designed specifically for medical devices. Based on past experience, ISO 13485:2003 is preferred by TFDA for QSD. Once QSD is in compliance with regulations, TFDA will issue a QSD certificate, which is valid for three years. Also, foreign medical device manufacturers must obtain a Certificate to Foreign Government (CFG) proving that the device is cleared or approved for sale in their original countries. Both the QSD and the CFG should be included for premarket approval and registration.
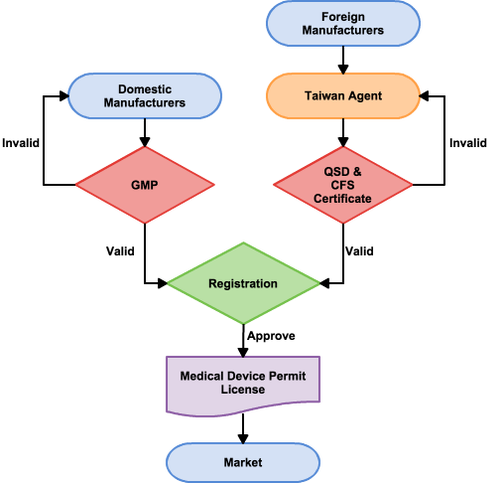
Along with these certificates, other information including device information, product test report, clinical testing date (if applicable) and more must be submitted to the TFDA. Testing conducted outside of Taiwan is usually accepted by the TFDA. If the TFDA approves the device registration, a Medical Device Permit License will be issued to the applicant. Then, the device is ready for sale in Taiwan. Keep in mind that the registration is valid for 5 years, and renewal documents must be submitted six months before expiration. Shown below is a flowchart about medical device registration process.
AcmeBiotechs Consulting offers services helping clients through registration processes as well as applying for GMP, QSD, CFS. We assist our clients in accessing the Taiwanese market without concerns about compliance with medical device regulations.
To apply for premarket approval, foreign medical device manufacturers are required to appoint an in-country representative, also known as a Taiwan Agent, to manage their device registration process and communicate with the Taiwan Food and Drug Administration (TFDA) on their behalf. A Taiwan Agent must have a local address in Taiwan and hold a formal license for medical device wholesale, medical device retail or international business. A Taiwan Agent should be responsible for submitting all documentation required for device approval and registration processes.
During the approval application, medical companies need to have quality system documents, known as QSD, to prove that the manufacturing steps comply with the FDA Quality System Regulation or ISO 13485:2003. This is a quality system standard designed specifically for medical devices. Based on past experience, ISO 13485:2003 is preferred by TFDA for QSD. Once QSD is in compliance with regulations, TFDA will issue a QSD certificate, which is valid for three years. Also, foreign medical device manufacturers must obtain a Certificate to Foreign Government (CFG) proving that the device is cleared or approved for sale in their original countries. Both the QSD and the CFG should be included for premarket approval and registration.
Along with these certificates, other information including device information, product test report, clinical testing date (if applicable) and more must be submitted to the TFDA. Testing conducted outside of Taiwan is usually accepted by the TFDA. If the TFDA approves the device registration, a Medical Device Permit License will be issued to the applicant. Then, the device is ready for sale in Taiwan. Keep in mind that the registration is valid for 5 years, and renewal documents must be submitted six months before expiration. Shown below is a flowchart about medical device registration process.
AcmeBiotechs Consulting offers services helping clients through registration processes as well as applying for GMP, QSD, CFS. We assist our clients in accessing the Taiwanese market without concerns about compliance with medical device regulations.