China
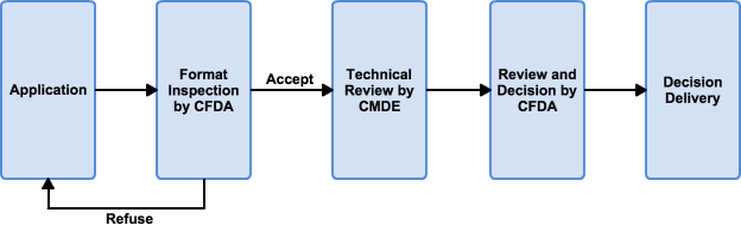
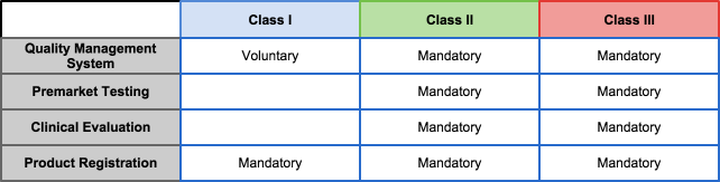
Foreign medical device manufacturers planning to access the Chinese market have to comply with the Chinese Food and Drug Administration (CFDA) regulations and acquire CFDA approval. These regulations are implemented in "Regulations for Supervision and Administration of Medical Devices" and "Provisions for Medical Device Registration." Similar to many other countries, the CFDA classifies medical devices into Class I, Class II, or Class III based on the level of risk, terms of use, and intended use. Devices of all classes will be inspected and granted approval with a registration certificate by the CFDA. Clinical evaluation must be conducted for Class II and Class III medical devices. Clinical evaluations and trials need to be conducted in China by certificated laboratories and institutions. For imported medical devices, their initial registrations can be divided into three conditions:
In each condition, the manufacturers have to provide different reports and documents to show that their products are safe and effective for their intended use. Also, the CFDA requires all medical device companies to designate a registered official agent (China Agent) to report to the CFDA on the behalf of the companies. With sufficient materials, and after pass the CFDA review, the CFDA will issue a marketing license which is valid for 5 years.
AcmeBiotechs helps our clients prepare the CFDA application and device registration and find China Agents. We can help our clients grow and explore the Chinese market without hindrance from complex but important regulations. Contact us for any questions related to entering the Chinese medical device market!
- devices that are approved for marketing abroad
- Class I devices that are not certified abroad,
- Class II and III devices that are not certified abroad
In each condition, the manufacturers have to provide different reports and documents to show that their products are safe and effective for their intended use. Also, the CFDA requires all medical device companies to designate a registered official agent (China Agent) to report to the CFDA on the behalf of the companies. With sufficient materials, and after pass the CFDA review, the CFDA will issue a marketing license which is valid for 5 years.
AcmeBiotechs helps our clients prepare the CFDA application and device registration and find China Agents. We can help our clients grow and explore the Chinese market without hindrance from complex but important regulations. Contact us for any questions related to entering the Chinese medical device market!